
Our history
“We try never to forget that medicine is for the people. It is not for the profits.”
– George Merck
For over 130 years, we've been guided by the view that great medicines and vaccines change the world.
Our legacy of inventing medicines and vaccines continues to this day. We adapt our business not only for the next quarter but for the next quarter century.

1891
Founding
Merck & Co., Inc. Rahway, NJ U.S. was founded on January 1, 1891. George Merck, age 23, established the company in the U.S. to distribute fine chemicals throughout New York City and the neighbouring areas. Merck & Co., Inc., Rahway, New Jersey, U.S. is known as MSD everywhere outside of the U.S. and Canada.

1899
The first Manual published
Our company first published the book entitled The Merck Manual in the U.S. in 1899 (now known as The MSD Manual outside the U.S. and Canada). Treatments in the first manual included bloodletting for acute bronchitis, arsenic for impotence and almond bread for diabetes. The Manual went on to become one of the most widely used medical references in the U.S..

1933
The first Research Laboratory created
The MRL Research Laboratory was founded in Rahway, New Jersey. The laboratory represented the initial foray into pharmacological research and included three separate divisions: Pure Research, the Institute for Therapeutic Research and Applied Research.

1940s
Discovered and distributed breakthrough antibiotic, streptomycin
Tuberculosis was historically a leading cause of death in the U.S. In 1943, Dr. Selman Waksman and Albert Schatz discovered streptomycin, the first effective treatment for the disease. MSD had supported Dr. Waksman’s research lab and held the new drug’s patent rights. Once its significant health benefits were recognized, MSD relinquished its exclusive patent on the antibiotic to ensure maximum patient access.

1946
MSD succeeds in first production of cortisone
After doctors at the Mayo Clinic discovered cortisone, it was MSD scientist Dr. Lewis Scarett who made the commercial production of cortisone possible – thanks to the 37-step chemical synthesis process he developed. Cortisone was the first corticosteroid to be used as a drug. Today, corticosteroids are still among the ten most widely used drugs in the world.

1953
Merger with Sharp & Dohme
The merger to create Merck Sharp & Dohme (MSD) [(headquartered in Rahway, New Jersey, U.S.)] brought together MSD’s extensive chemical research and manufacturing facilities with Sharp & Dohme’s pharmaceutical development, marketing expertise and international presence. Sharp & Dohme’s West Point, Pennsylvania facilities were included in the merger.

1963
First Swiss Subsidiary founded
The first Swiss subsidiary of MSD was founded in Zurich.

1963
MSD launches the first vaccine against measles
Vaccine development at MSD is strongly associated with the name of Dr. Maurice Hilleman, who has been involved in the development of more than 35 vaccine products in his more than 25 years with the company. The measles vaccine alone has saved about 125 million lives since 1963. In addition to the first measles vaccine, MSD also introduced the first mumps and rubella vaccine (1967 and 1969) – as well as the first mumps-measles-rubella triple vaccine (1971).
1977
First pneumonia vaccine was approved
MSD’s pneumonia vaccine, was approved. Research and development of the vaccine was carried out under the direction of Dr. Maurice Hilleman.

1987
MSD committed to donate Mectizan – as much as needed for as long as needed – with the goal to eliminate river blindness.
In 1987, CEO Dr. Roy Vagelos announced MSD’s commitment to donate Mectizan – as much as needed for as long as needed – with the goal to help eliminate river blindness. In order to reach this goal, our leaders recognized that many organizations with unique skills would need to work together as a team. Thus, the Mectizan Donation Program (MDP) was created as a groud-breaking public-private partnership that becomes influential in the development of a number of other drug donation programs.

1987
MSD introduced the first commercial statin
We introduced the first of a statin family of medicines to be approved by the FDA. It emerged from decades of study by scientists, including ours, around the world.

1996
FDA grants MSD accelerated approval of MSD drug for the treatment of HIV
The U.S. Food and Drug Administration (FDA) granted approval for the MSD compound in a record time of 42 days. At the time of its approval, the drug was one of the most effective antiviral agents. It was instrumental in making HIV a survivable infection and paving the way for combination therapy.

2006
Significant therapeutic progress in diabetes therapy
MSD not only launched the first DPP-4 inhibitor, but also the first new oral therapy for type 2 diabetes in more than a decade. For the discovery, Ann E. Weber and Nancy A. Thornberry received the Discoverers’ Award from PhRMA (Pharmaceutical Research and Manufacturers of America).

2006
Approval of the first vaccine against cervical cancer
The FDA approved MSD’s vaccine for the prevention of cervical cancer caused by certain HPV types.
In 2007, MSD committed to donating 3 million doses over 5 years to support vaccination programs in the world’s lowest-income nations.

2009
Merger with Schering-Plough
MSD and Schering-Plough completed a merger and began combined operations. The Swiss subsidiaries Essex Chemie AG and MSD Switzerland were merged in 2011 and since then had the name MSD Merck Sharp & Dohme AG. The former location of Essex Chemie AG in Lucerne became the headquarters of the merged companies.

2011
Global MSD for Mothers initiative began
In 2010, one woman died every two minutes during childbirth and pregnancy. Many of these deaths were preventable. In response, we launched MSD for Mothers, a global initiative with partners to improve the health and well-being of women before, during and after pregnancy and childbirth. As of 2019, the effort has reached more than nine million women globally in 48 countries.

2014
Accelerated approval of a new immuno-oncology therapy
The FDA approved the first anti-PD-1 (programmed death receptor-1) therapy, that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. In Switzerland, the therapy received its first approval in 2015. Until today MSD has the industry’s largest immuno-oncology clinical research program with currently more than 1,200 clinical trials in a wide variety of cancers and treatment settings (as of 05/2021).
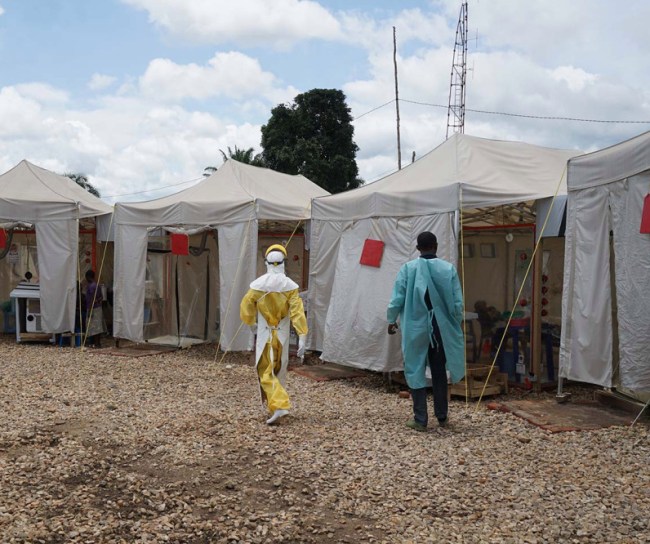
2019
MSD received FDA approval for Ebola Zaire Vaccine
From Guinea to the Democratic Republic of the Congo (DRC), the world was dealing with the largest and most complex Ebola outbreaks since the virus was first discovered in 1976. As the outbreaks remained a global health challenge, MSD scientists, along with numerous external collaborators from all sectors, remained at the forefront of the efforts to address this deadly disease.
CH-NON-00880, 04/2023