Klinische Studien
“Klinische Studien sind der Schlüssel zu neuen Erkenntnissen in der Diagnostik und Therapie von Erkrankungen. Wir sind stolz, in der Schweiz dazu beizutragen.”
Klaudia Georgi, Sr. Director Clinical Research MSD Switzerland & Austria
Unsere Arbeit in Zahlen
Laufende klinische Studien in der Schweiz (Stand 01/2025)
In der Schweiz zugelassene First-in-Class-Medikamente und Impfstoffe seit 2000 (Stand 01/2025)
Millionen Schweizer Franken in die Forschung investiert, seit 2012 (Stand 01/2025)

Die klinische Forschung von MSD Schweiz wird in unserer Niederlassung im Citybay in Luzern koordiniert
Das klinische Studienprogramm von MSD in der Schweiz (Stand 01/2025)
(veröffentlicht auf www.clinicaltrials.gov)
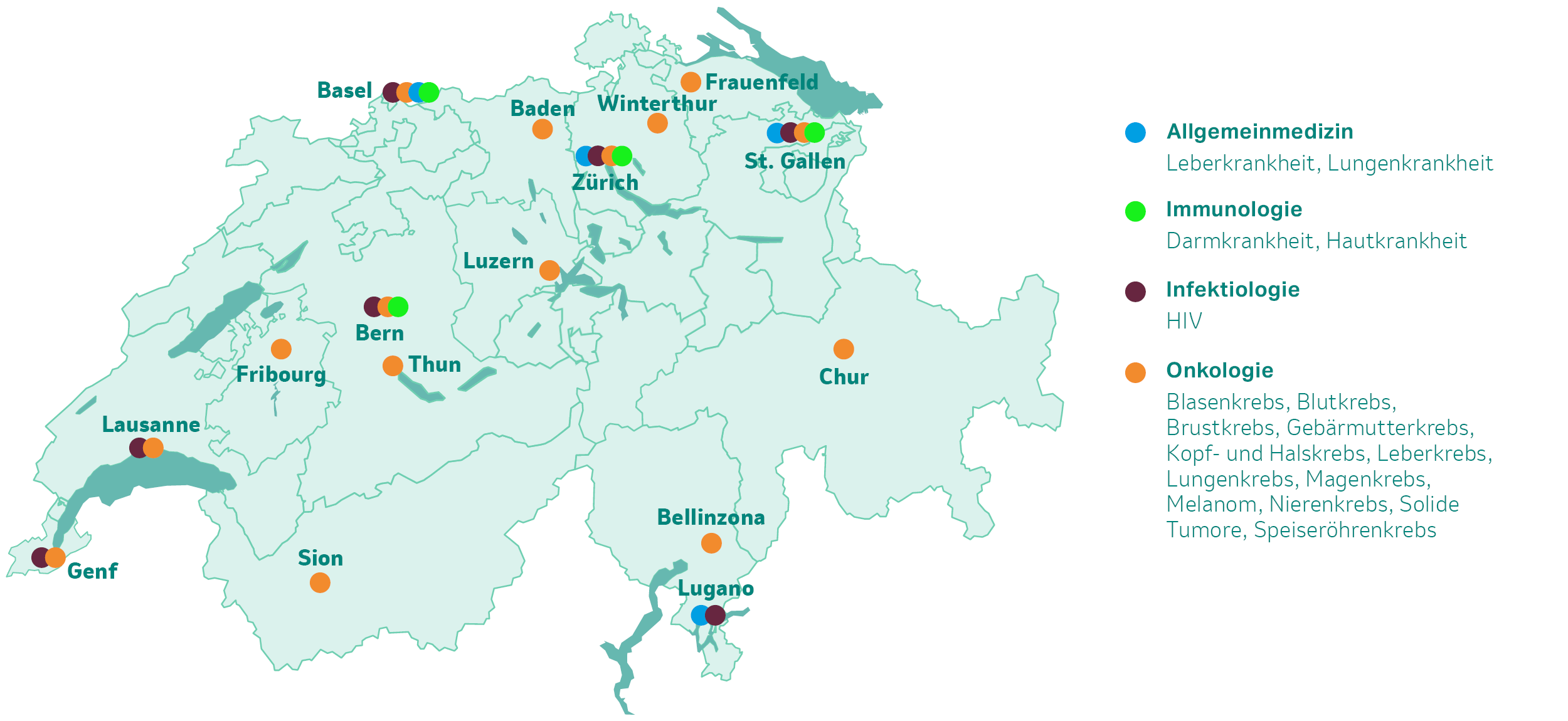
Im Bereich Allgemeinmedizin
Leberkrankheit
MK-6024-013 – recruitment closed
A Phase 2b Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Efinopegdutide (MK-6024) in Adults with Precirrhotic Nonalcoholic Steatohepatitis (NASH)
Study centers: Lugano, St. Gallen, Zurich
Lungenkrankheit
MK-5475-013 – recruitment open
A Phase 2a Randomized, Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of MK-5475 in Adults With Pulmonary Hypertension Associated With Chronic Obstructive Pulmonary Disease
Study centers: St. Gallen, Zurich, Basel
Im Bereich Immunologie
Darmkrankheit
MK-7240-001 – recruitment open
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Program to Evaluate the Efficacy and Safety of MK-7240 in Participants with Moderately to Severely Active Ulcerative Colitis
Study centers: Bern, St.Gallen, Zurich
MK-7240-008 – recruitment open
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Program to Evaluate the Efficacy and Safety of MK-7240 in Participants with Moderately to Severely Active Crohn’s Disease
Study centers: Basel, Bern, St.Gallen, Zurich
MK-7240-011 – study in start-up
A Phase 3 Extension Study to Evaluate the Long-term Safety and Efficacy of Tulisokibart in Participants With Crohn’s Disease or Ulcerative Colitis
Study centers: Basel, Bern, St.Gallen, Zurich
Hautkrankheit
MK-6194-007 – recruitment closed
A Phase 2a, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of MK-6194 in Adult Participants with Non-Segmental Vitiligo
Study centers: St.Gallen, Zurich
Im Bereich Infektiologie
HIV
MK-8591-013 – recruitment closed
A Phase 2b, Randomized, Active-Controlled, Double-Blind, Dose-Ranging Clinical Study to Evaluate a Switch to Islatravir (ISL) and MK-8507 Once-Weekly in Adults with HIV-1 Virologically Suppressed on Bictegravir/Emtricitabine/Tenofovir Alafenamide (BIC/FTC/TAF) Once Daily
Study centers: Basel, Bern, Lausanne
MK-8591A-051 – recruitment closed
A Phase 3, Randomized, Active-Controlled, Open-Label Clinical Study to Evaluate a Switch to Doravirine/Islatravir (DOR/ISL 100 mg/0.25 mg) Once-Daily in Participants With HIV-1 Who Are Virologically Suppressed on Antiretroviral Therapy
Study centers: Basel, Bern, Geneva, Lausanne, Lugano, Zurich
MK-8591A-053 – recruitment closed
A Phase 3, Randomized, Active-Controlled, Double-Blind Clinical Study to Evaluate the Antiretroviral Activity, Safety, and Tolerability of Doravirine/Islatravir (DOR/ISL 100 mg/0.25 mg) Once-Daily in HIV-1 Infected Treatment-Naïve Participants
Study centers: Basel, Geneva
MK-8591A-054 – recruitment closed
A Phase 3 Open-label Clinical Study of Doravirine/Islatravir (DOR/ISL [100 mg/0.25 mg]) Once Daily for the Treatment of HIV-1 Infection in Participants Who Previously Received DOR/ISL (100 mg/0.75 mg) QD in a Phase 3 Clinical Study
Study centers: Basel, Bern, Geneva, St. Gallen, Lugano, Zurich
MK-8591B-060 – study in start-up
A Phase 2b, Randomized, Active-Controlled, Open-Label Clinical Study to Evaluate a Switch to Islatravir (ISL) and Ulonivirine (ULO) Once-Weekly in Adults with HIV-1 Virologically Suppressed on Bictegravir/Emtricitabine/Tenofovir Alafenamide (BIC/FTC/TAF) Once-Daily
Study centers: Basel, Bern, Geneva, Lugano
Im Bereich Onkologie
Blasenkrebs
MK-3475-676/KEYNOTE-676 – recruitment closed
A Phase 3, Randomized, Comparator-controlled Clinical Trial to Study the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination with Bacillus Calmette-Guerin (BCG) in Participants with High-risk Non-muscle Invasive Bladder Cancer (HRNMIBC) that is Persistent or Recurrent Following BCG Induction (KEYNOTE-676)
Study centers: Basel, Geneva, Zurich
Blutkrebs (Hämatologische Malignome)
MK-1026-003 – recruitment open
A Phase 2 Study to Evaluate the Efficacy and Safety of MK-1026 in Participants with Hematologic Malignancies
Study centers: Bellinzona, Bern
MK-2140-010 – study in start-up
A Randomized, Open-Label, Multicenter, Phase 3 Study of Zilovertamab Vedotin (MK-2140) in Combination With R-CHP Versus R-CHOP in Participants With Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL) (waveLINE-010)
Study centers: Baden, Bellinzona, St.Gallen
MK-4280A-008 – recruitment closed
A Phase 3 Randomized Clinical Study of MK-4280A (coformulated favezelimab [MK-4280] plus pembrolizumab [MK-3475]) Versus Physician’s Choice Chemotherapy in PD-(L)1-refractory, Relapsed or Refractory Classical Hodgkin Lymphoma (KEYFORM-008)
Study centers: Bellinzona
Brustkrebs
MK-2870-010 – recruitment open
An Open-label, Ranomized Phase 3 Study of MK-2870 as a Single Agent and in Combination with Pembrolizumab Versus Treatment of Physician`s Choice in Participants with HR+/HER2- Unresectable Locally Advanced or Metastatic Breast Cancer
Study centers: Basel, Thun, Zurich
MK-2870-012 – recruitment open
A Phase 3, Randomized, Open-label, Study to Compare the Efficacy and Safety of Adjuvant MK-2870 in Combination with Pembrolizumab (MK-3475) Versus Treatment of Physician’s Choice in Participants With Triple Negative Breast Cancer (TNBC) Who Received Neoadjuvant Therapy and Did Not Achieve a Pathological Complete Response (pCR) At Surgery
Study centers: Chur, Fribourg, Geneva, Thun, Zurich
Gebärmutterkrebs
MK-2870-005 – recruitment open
A Phase 3, Randomized, Active-controlled, Open-label, Multicenter Study to Compare the Efficacy and Safety of MK-2870 Monotherapy Versus Treatment of Physician’s Choice in Participants With Endometrial Cancer Who Have Received Prior Platinum-based Chemotherapy and Immunotherapy
Study centers: Bern, Bellinzona, Chur, Lausanne, Basel
MK-2870-020 – recruitment open
A Phase 3 Randomized, Active-controlled, Open-label, Multicenter Study to Compare the Efficacy and Safety of MK-2870 Monotherapy Versus Treatment of Physician’s Choice as Second-line Treatment for Participants with Recurrent or Metastatic Cervical Cancer
Study centers: Basel, Bern
Kopf- und Halskrebs
A Phase III, Randomized, Open-label Study to Evaluate Pembrolizumab as Neoadjuvant Therapy and in Combination With Standard of Care as Adjuvant Therapy for Stage III-IVA Resectable Locoregionally Advanced Head and Neck Squamous Cell Carcinoma (LA HNSCC)
Study centers: Geneva
Leberkrebs
MK-3475-937/KEYNOTE-937 – recruitment closed
A Phase 3 Double-blinded, Two-arm Study to Evaluate the Safety and Efficacy of Pembrolizumab (MK-3475) versus Placebo as Adjuvant Therapy in Participants with Hepatocellular Carcinoma and Complete Radiological Response after Surgical Resection or Local Ablation
Study centers: Basel, Bern, Geneva, Lausanne, St. Gallen, Winterthur, Zurich
MK-1308A-004 – recruitment closed
A Phase 2, Multicenter, Clinical Study to Evaluate the Safety and Efficacy of MK-1308A (Coformulated MK-1308/MK-3475) in Combination with Lenvatinib (E7080/MK-7902) in First-line Therapy of Participants with Advanced Hepatocellular Carcinoma
Study centers: Bern, Geneva, Lausanne, Zurich
Lungenkrebs
MK-2870-019 – recruitment open
A Phase 3 Randomized Open-label Study of Adjuvant Pembrolizumab with or without MK-2870 in Resectable Stages II-IIIB (N2) NSCLC for participants not achieving pCR after Receiving Neoadjuvant Pembrolizumab with Platinum-based Doublet Chemotherapy Followed by Surgery
Study centers: Lausanne, Fribourg, Frauenfeld, Chur
MK-3475-091/KEYNOTE-091 – outsourced – recruitment closed
A randomized, phase 3 trial with anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy (PEARLS)
Study centers: Basel, Bellinzona, Bern, Chur, Geneva, Lausanne, Lucerne, St. Gallen, Winterthur, Zurich
MK-3475-495/KEYNOTE-495; KeyImPaCT – recruitment closed
KeyImPaCT: A Phase 2 Precision Oncology Study of Biomarker-Directed, Pembrolizumab-(MK-3475, SCH 900475) Based Combination Therapy for Advanced Non-Small Cell Lung Cancer
Study centers: Basel, St. Gallen, Zurich
MK-3475-B98 – recruitment closed
A Phase 1b/2 Study to Evaluate the Efficacy and Safety of Pembrolizumab in Combination with Investigational Agents for the Treatment of Participants With PD-1/L1-refactory Extensive Stage Small Cell Lung Cancer in Need of Second-Line Therapy
Study center: St. Gallen
Magenkrebs
MK-1022-011 – study in start-up
A Phase 1/2 Study to Evaluate the Safety and Efficacy of Patritumab Deruxtecan in Gastrointestinal Cancers
Study centers: Geneva, Zurich
MK-3475-859/KEYNOTE-859 – recruitment closed
A Phase III, randomized, double-blind clinical study of pembrolizumab (MK-3475) plus chemotherapy versus placebo plus chemotherapy as first line treatment in participants with previously untreated, HER2 negative, advanced gastric or gastroesophageal junction adenocarcinoma
Study centers: Bellinzona
MK-5909-005 – study in start-up
A Phase 2 Nonrandomized, Open-label, Multisite Study to Evaluate the Safety and Efficacy of Raludotatug Deruxtecan in Participants With Gastrointestinal Cancers
Study centers: Basel, Chur, Geneva
MK-9999-U02A – recruitment open
A Phase 1/2 Substudy of the MK-9999-U02 Master Protocol to Evaluate the Safety and Efficacy of MK-2870 Monotherapy or in Combination With Other Anticancer Agents in Gastrointestinal Cancers
Study centers: Bellinzona, Geneva
Melanom (Schwarzer Hautkrebs)
MK-3475-U02A – recruitment open
A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants with Melanoma (KEYNOTE-U02) – refractory
Study centers: Geneva, Zurich
MK-3475-U02B – recruitment open
A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants with Melanoma (KEYNOTE-U02) – 1st line
Study centers: Geneva, Zurich
MK-3475-U02C – recruitment open
A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants with Melanoma (KEYNOTE-U02) – neoadjuvant
Study centers: Geneva, Zurich
MK-3475-U02D – recruitment open
A Phase 1/2 Open-Label Rolling-Arm Umbrella Platform Design of Investigational Agents With or Without Pembrolizumab or Pembrolizumab Alone in Participants with Melanoma (KEYNOTE-U02) – brain metastasis
Study centers: Geneva, Zurich
MK-3475-054 / KEYNOTE-054 – outsourced – recruitment closed
Adjuvant immunotherapy with anti-PD-1 monoclonal antibody Pembrolizumab (MK-3475) versus placebo after complete resection of high-risk Stage III melanoma: A randomized, double-blind Phase 3 trial of the EORTC Melanoma Group
Study centers: Geneva, St. Gallen, Zurich
MK-3475-716/KEYNOTE-716 – recruitment closed
Adjuvant Therapy with Pembrolizumab Versus Placebo in Resected High-risk Stage II Melanoma: A Randomized, Double-blind Phase 3 Study
Study centers: Bellinzona, Basel, Bern, Chur, Geneva, Lausanne, Sion, St. Gallen, Zurich
MK-7684A-010 – recruitment closed
A Phase 3, Randomized, Double-blind, Active-Comparator-Controlled Clinical Study of Adjuvant MK‑7684A (Vibostolimab with Pembrolizumab) Versus Adjuvant Pembrolizumab in Participants with High-risk Stage II-IV Melanoma
Study centers: St.Gallen, Zurich, Bern, Basel, Lausanne, Geneva, Sion, Bellinzona
Nierenkrebs (Nierenzellkarzinom)
MK-6482-011 – recruitment closed
An Open-label, Randomized, Phase 3 Study of MK-6482 in Combination with Lenvatinib (MK-7902) vs Cabozantinib for Second-line or Third-line Treatment in Participants with Advanced Renal Cell Carcinoma Who Have Progressed After Prior Anti-PD-1/L1 Therapy
Study centers: Bellinzona, Chur, Geneva, Zurich
Solide Tumore
MK-0472-001 – recruitment open
A Phase 1/1b Open-label, Multicenter Clinical Study of MK-0472 as Monotherapy and Combination Therapy in Participants with Advanced/Metastatic Solid Tumors.
Study centers: Geneva, Bellinzona, St. Gallen
MK-1084-001 – recruitment open
A Phase 1, Open-Label, Multicenter Study to Assess Safety, Tolerability, PK, and Efficacy of MK-1084 as Monotherapy and in Combination with Pembrolizumab in Subjects with KRASG12C Mutant Advanced Solid Tumors
Study centers: Bellinzona, St. Gallen
MK-3475-587/KEYNOTE-587 – recruitment closed
Long-term Safety and Efficacy Extension Study for Participants with Advanced Tumors Who Are Currently on Treatment or in Follow-up in a Pembrolizumab (MK-3475) Study
Study center: Basel, Geneva, Zurich
MK-6598-001 – recruitment closed
A Phase I, Open-label, Multicenter Study to Assess Safety, Tolerability, PK, and Efficacy of MK-6598 as Monotherapy and in Combination With Pembrolizumab in Participants With Advanced Solid Tumors
Study centers: Bellinzona, Geneva, St. Gallen
MK-7339-002 – recruitment closed
A Phase 2 Study of Olaparib Monotherapy in Participants with Previously Treated, Homologous Recombination Repair Mutation (HRRm) or Homologous Recombination Deficiency (HRD) Positive Advanced Cancer
Study center: Bellinzona
Speiseröhrenkrebs
MK-3475-06A – recruitment closed
A Phase 1/2 Open-Label, Umbrella Platform Design Study of InvestigationalAgents With Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer naïve to PD-1/PD-L1 Treatment: Substudy 06A.
Study centers: Chur, Geneva
MK-3475-06B – recruitment open
A Phase 1/2 Open-Label, Umbrella Platform Design Study of Investigational Agents With Pembrolizumab (MK-3475) in Participants With Advanced Esophageal Cancer Previously Exposed to PD-1/PD-L1 Treatment: Substudy 06B.
Study centers: Chur, Geneva
MK-3475-06C – recruitment open
A Phase 1/2 Open-Label, Umbrella Platform Design Study of MK-2870 With Pembrolizumab (MK-3475) and Chemotherapy in Participants With 1L Locally Advanced Unresectable/ Metastatic Gastroesophageal Adenocarcinoma (Gastric Adenocarcinoma, Gastroesophageal Junction Adenocarcinoma, and Esophageal Adenocarcinoma): Substudy 06C
Study centers: Chur, Geneva
MK-3475-06D – recruitment open
A Phase 1/2 Open-Label, Umbrella Platform Design Study to Evaluate the Safety and Efficacy of MK-2870 Plus Paclitaxel as the Second-Line Treatment of Participants With Advanced/Metastatic Gastroesophageal Adenocarcinoma: Substudy 06D
Study centers: Chur, Geneva
MK-3475-06E – study in start-up
A Phase 1/2 Open-Label, Umbrella Platform Design Study of Investigational Agents in Combination With Pembrolizumab (MK-3475) With or Without Chemotherapy in Participants With 1L Locally Advanced Unresectable/Metastatic Esophageal Cancer: Substudy 06E
Study centers: Chur, Geneva
Zur Übersicht der schweizweiten Studien, die Teilnehmende suchen www.kofam.ch
CH-NON-00136, 01/2025